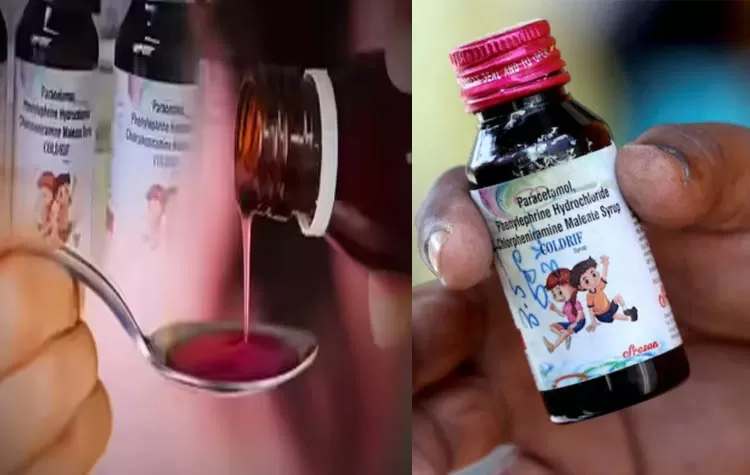
Background of the Incident
Over 20 children have lost their lives in Madhya Pradesh after taking the Coldriff cough syrup. The drug had been manufactured by Sresan Pharma, a pharmaceutical firm based in Kanchipuram, Tamil Nadu. During an inspection, officials found that the syrup contained 48.6% diethylene glycol, a toxic substance. Subsequent to this discovery, authorities initiated a thorough probe into the company's operations.
ED Searches in Chennai
On Monday, officials of the Enforcement Directorate (ED) conducted raids at seven premises in Chennai linked to Sresan Pharma. It was reported that searches were also carried out at the residences of top officials of the Tamil Nadu Drug Control Department. These operations were after Ranganathan (73), the proprietor of Sresan Pharma, was arrested.
Negligence of Regulatory Authorities
Probers discovered that the Tamil Nadu drug officials did not inspect the firm, even a single time, since its inception. Their inaction is implicated in the fatalities due to the poisonous syrup. The Central Drug Control Organization (CDSCO), as per national media reports, found several failings during its investigation into the Coldriff case.
Key Findings of the Investigation
Sources uncovered that the state drug regulatory agency disowned key guidelines and failed to act on notices laid down by the Center. No monitoring of the process for making the syrup was carried out in due time. As a result of inadequate supervision in time, infected cough syrup found its way into the market, resulting in numerous deaths of children.
Regulatory Failures and Compliance Issues
Investigators further found that not a single government department had audited Sresan Pharma. Even the firm was not registered on the central pharmaceutical portal as a manufacturer of authorized drugs. In spite of all these anomalies, the Tamil Nadu government permitted the company to function for decades without acquiring a Good Manufacturing Practice (GMP) certificate from the World Health Organization (WHO).













